The bonding between the Metal and the CO ligand is both a σ and π interaction. The ligand's HOMO donates electrons to the metal's orbitals to form the σ bond. Because the ligand's LUMO is a π molecular orbital, the metal's orbitals can donate electrons back into the ligand's LUMO. This is called back-bonding because the metal's orbitals donate electrons back to the ligand.
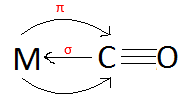 Because CO can have metal back-bonding, it is called a π-acceptor ligand (because the ligand accepts electrons into its π molecular orbitals from the metal). | |
| Back | Next |
|---|---|