Because the LUMO is not a π molecular orbital, the metal does not back-bond into O2. Instead, O2's π molecular orbitals also donate electrons to the metal's orbitals once again making the interaction between the Metal and the O2 ligand is both a σ and π interaction:
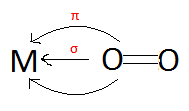 Because O2 cannot have back-bonding from the metal (because it has full π molecular orbitals), it is called a π-donor ligand (its π molecular orbitals donate electrons to the metal rather than the metal back-bonding to the ligand). | |
| Back | Next |
|---|---|